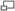Second Law of Thermodynamics
An introduction to the second law of thermodynamics, and discussing Carnot's theorem.
Overview
Definitions
Heat engine is any machine that can convert heat into work and vice versa.
Available energy is the proportion of heat supplied to a machine which can be converted into mechanical work. This proportion is found to be dependent on the temperature of the heat source, and also on the temperature at which heat is rejected.
<br/>
Key facts
The second law of thermodynamics states that heat can not be transferred from a body at a lower temperature to a body at a higher temperature without the application of energy from an external source.
Carnot's theorem states that no machine can be more efficient than a machine working under a thermodynamically reversible cycle, provided that they are both working under the same limits of temperature.
Carnot's theorem can be proved by reductio ad absurdum using the second law of thermodynamics.
The second law of thermodynamics states that heat can not be transferred from a body at a lower temperature to a body at a higher temperature without the application of energy from an external source.
Carnot's Theorem
The second law of thermodynamics can be used to prove Carnot's theorem. However, before discussing Carnot's theorem, we first have to introduce the thermal efficiency of a heat engine. The thermal efficiency of a heat engine, also denoted by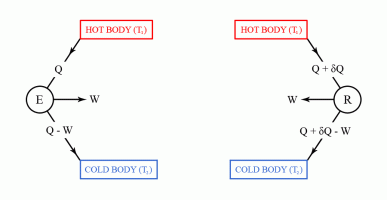

 Login
Login